As nicotine pouches continue to attract regulatory attention, discussion has increasingly focused on isolated clinical observations and precautionary warnings, often without sufficient context on real-world use patterns or comparative risk. This imbalance risks obscuring a more important question for public health: how nicotine pouches are actually used, by whom, and how regulation can address legitimate safety concerns without undermining smoking cessation and harm reduction.
Three areas in particular remain underexplored in policy debates: oral health findings, uptake data, and the regulatory implications of both.
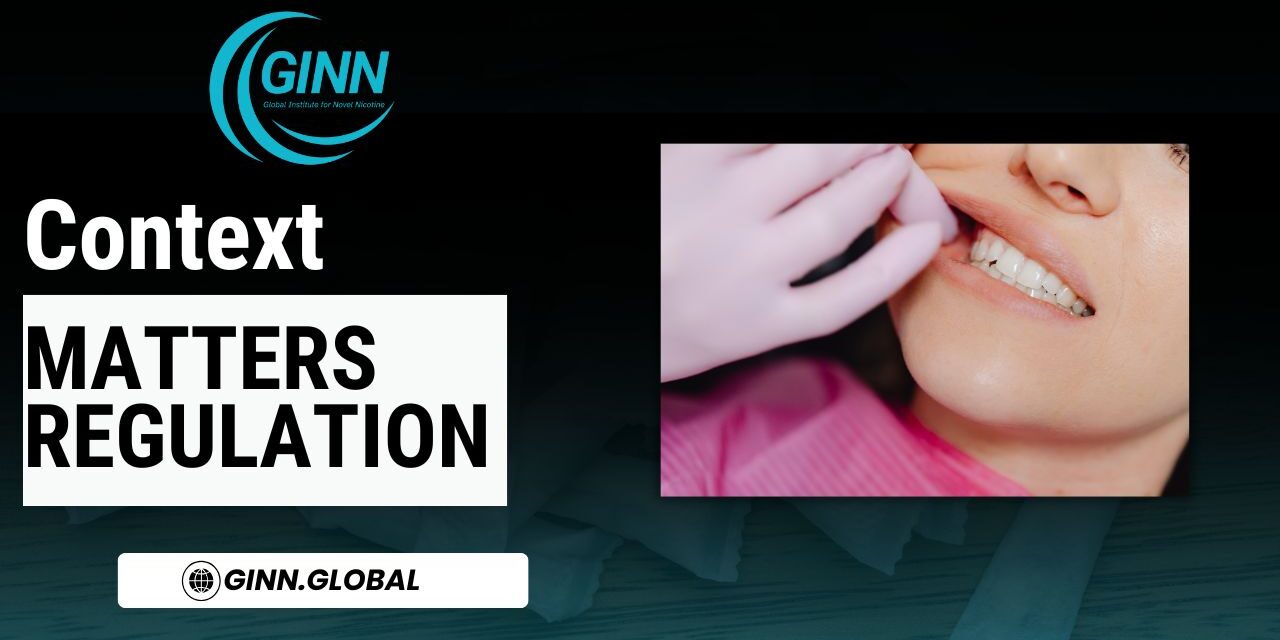
Oral lesions: signal, not verdict
Clinical reports have documented oral mucosal changes among some nicotine pouch users, including localized irritation and reversible lesions at the site of placement. These findings are not surprising. Similar transient oral effects have long been observed with other oral nicotine products, including traditional snus and licensed nicotine replacement therapies such as gum and lozenges.
Crucially, the available evidence does not show that these oral changes translate into serious pathology or long-term disease outcomes. Histological assessments in related smokeless and oral nicotine research consistently distinguish between local irritation and systemic harm, the latter being overwhelmingly driven by combustion products rather than nicotine itself.
From a regulatory standpoint, these observations argue for product standards, user guidance, and post-market surveillance, not for categorical conclusions about harm or blanket restriction.
Uptake data: who is actually using nicotine pouches?
Population-level evidence paints a clearer picture of who uses nicotine pouches and why. Across multiple jurisdictions, uptake remains concentrated among current and former smokers, with use among nicotine-naïve adults and never-smokers remaining low.
This pattern matters. It suggests that nicotine pouches are functioning primarily as substitution tools, not as a new entry pathway into nicotine use. For adult smokers seeking alternatives to cigarettes, the appeal lies in the absence of combustion, discretion of use, and avoidance of smoke inhalation, factors directly relevant to reducing smoking-related harm.
Ignoring these uptake patterns risks regulatory decisions being made on hypothetical risks rather than observed behaviour.
The regulatory gap
Despite growing evidence, nicotine pouches are often regulated through frameworks designed either for combustible tobacco or for pharmaceuticals, neither of which fits their risk profile or consumer use.
This misalignment creates several problems:
- Over-regulation, where pouches are treated as equivalent to cigarettes despite vastly different toxicant exposure.
- Under-regulation, where lack of tailored rules leaves gaps in product standards, labelling, and oral health guidance.
- Confused public messaging, reinforcing the false perception that all nicotine products carry similar risks.
A proportionate framework would acknowledge nicotine pouches as non-combustible consumer products with lower toxicant exposure than smoking, while still addressing legitimate concerns around youth access, nicotine strength, and oral safety.
What proportionate regulation should look like
An evidence-aligned approach would include:
- Clear age restrictions and enforcement to protect minors
- Product standards covering ingredients, pH, and nicotine levels
- Labelling and guidance on appropriate use and oral placement
- Ongoing monitoring of oral health outcomes and use patterns
- Explicit differentiation between smoking and smoke-free nicotine in public communication
Such measures would strengthen consumer protection while preserving incentives for smokers to move away from combustible products.
A GINN perspective
For GINN, the conversation around nicotine pouches must move beyond fragmented signals and toward regulatory coherence. Oral health findings deserve attention, but they must be interpreted in context. Uptake data should guide policy, not be sidelined. And regulation should reflect relative risk, not default to equivalence with smoking.
If the objective is to reduce smoking-related disease, then frameworks must support, not obstruct, the transition away from combustion, while ensuring products on the market meet clear safety and quality standards.
Evidence, proportionality, and transparency remain the foundations of credible nicotine policy.